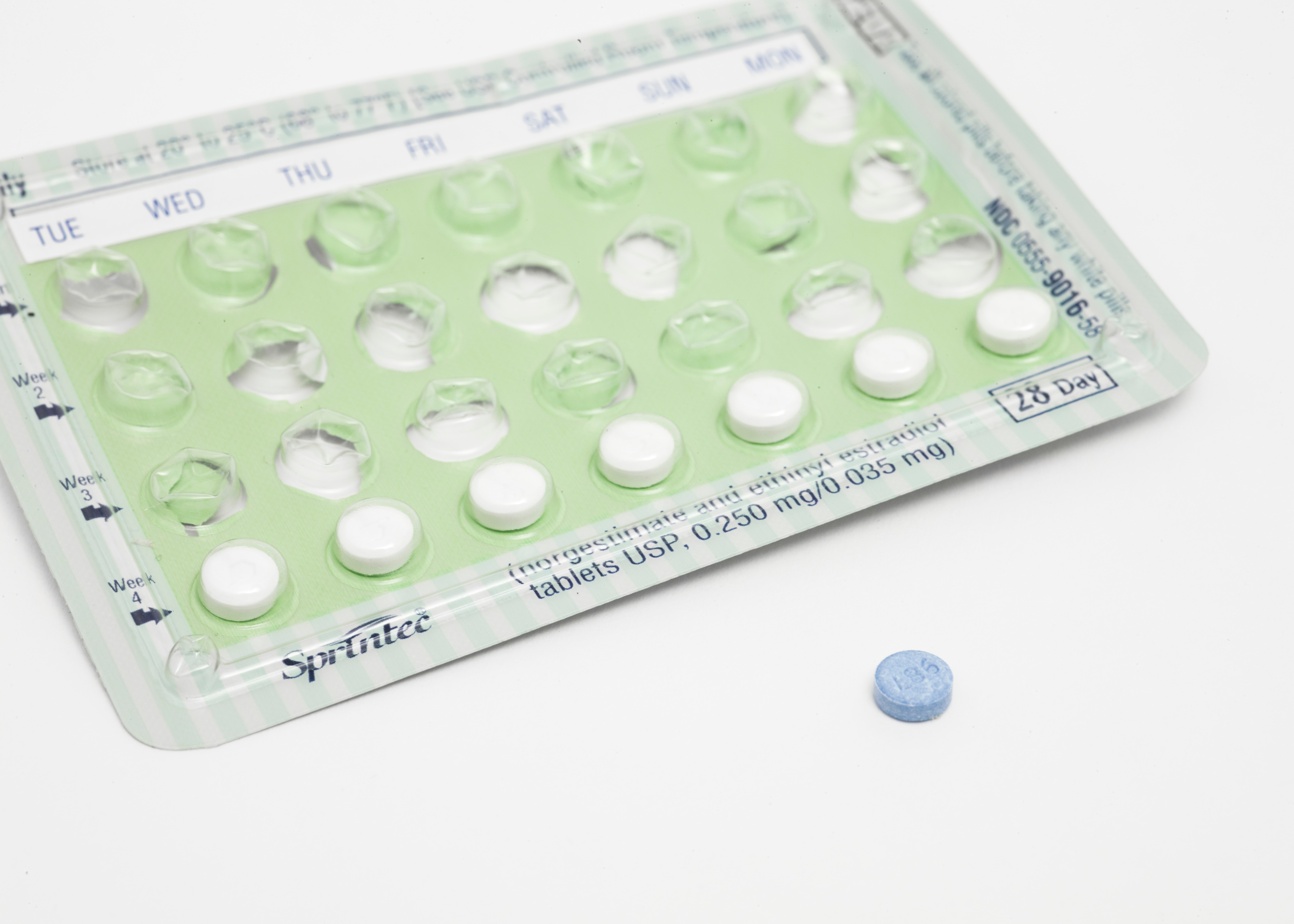
In an unprecedented move, the U.S. Food and Drug Administration (FDA) is deliberating over whether to allow over-the-counter (OTC) sale of birth control pills.
Kelly Blanchard, the leader of Ibis Reproductive Health, a nonprofit research organization, applauds the development as a significant landmark for contraceptive accessibility.
This week, the FDA is assembling a panel of independent consultants for a two-day meeting to assist in this decision-making process. The advisory panel will examine the scientific data and provide a recommendation, with the FDA expected to announce its final verdict by summer's end.
Making birth control pills readily available could greatly simplify the process for women. Historically, accessing these contraceptives has required a prescription, creating potential barriers for many women, according to Blanchard. She points out that lack of healthcare providers, scheduling, travel and appointment costs, taking time off work, and coordinating child care can all pose significant hurdles.
If approved, women could simply purchase birth control pills from any drugstore, a change that could particularly benefit women with limited resources.
The application under review is for a product named Opill, proposed by Perrigo. This contraceptive is a progestin-only pill, containing a synthetic version of the hormone progesterone to prevent pregnancy. Typically, birth control pills also contain estrogen.
Prominent medical organizations, including the American Medical Association and the American College of Obstetricians and Gynecologists, are supporting the proposition.
However, opposition exists, notably from groups like the Catholic Medical Association, and their objections extend beyond religious reasons. They raise concerns about the safety of OTC contraceptives, potential facilitation of sex trafficking, and the risk to women's health due to bypassing doctor's consultations. Dr. Timothy Millea of the association's healthcare policy committee argues that it may result in less screening for ovarian cancer, cervical cancer, and sexually transmitted infections.
The FDA's review also expresses concerns about excluding a healthcare professional from the process. They question the consistency of pill consumption and whether women with certain health conditions will be aware of the risks.
Supporters of the initiative counter these objections, citing evidence that women are capable of managing this responsibility. They point out that contraceptive pills are available OTC in over 100 countries.
Dr. Jack Resneck Jr., president of the AMA, assures that oral contraceptives have been used safely worldwide since the 1960s. He also clarifies that while regular medical examinations are crucial, they're not a prerequisite for starting or renewing an oral contraceptive.
In light of increasing restrictions on abortion access in the U.S., proponents argue that easy access to effective birth control is more crucial than ever.
Questions and Answers:
What is the FDA considering for the first time regarding birth control pills? The FDA is considering allowing over-the-counter sale of birth control pills for the first time in the U.S.
What could be a potential benefit of OTC birth control pills? Making birth control pills readily available could simplify the process for women by eliminating potential barriers such as lack of healthcare providers, scheduling, and costs related to appointments.
Which pill is currently being considered for OTC sale? The FDA is currently considering a contraceptive named Opill, proposed by Perrigo, for over-the-counter sale. This is a progestin-only pill that uses a synthetic form of the hormone progesterone to prevent pregnancy.
What are some objections to the OTC sale of birth control pills? Objections to the over-the-counter sale of birth control pills include concerns about the safety of these contraceptives, potential facilitation of sex trafficking, and risks to women's health due to bypassing doctor's consultations. There are also worries about missing out on important health screenings for conditions like ovarian and cervical cancer and sexually transmitted infections.
What is the counter-argument to these objections? Proponents of the initiative argue that there's ample evidence that women can manage this responsibility. They also highlight that oral contraceptives have been used safely worldwide since the 1960s, and while regular medical check-ups are important, they're not necessary before starting or renewing an oral contraceptive.
Why is easy access to effective birth control considered crucial now? In light of the increasing restrictions on abortion access in the U.S., easy access to effective birth control is considered more crucial than ever to ensure women can control their reproductive health.